Wrapping it up....Management
- Australian Society Dermal Clinicians

- Mar 5, 2021
- 7 min read
Updated: Mar 2, 2024
Chronic oedema and Lymphoedema are associated with significant impact on quality of life, due to the burden including on time, financial cost and psychosocial impact. Therefore prevention through education and identifying those at risk of lymphatic dysfunction is the goal. However the next aim is to recognise early signs of lymphatic impairment and implement management. Successful management is most effective with the use of a multidisciplinary approach. Healthcare models are beginning to implement centres of innovation and best practice that maximise the knowledge and skills of different disciplines in patient centered care. The management of lymphoedema lends itself to this approach. Dermal Clinicians should be aware of what therapies are available, what the evidence is for using these, what their role is within their scope of practice and where to send clients that need specialised assessment and management.

The Pillars of Management
Management of chronic oedema is comprised of 5 traditional pillars:
Education
Skin Management
Exercise
Compression Therapy
Manual Lymphatic Drainage
Used together these may also be referred to as complex decongestive therapy. Practitioners may also incorporate other therapies including kinesiotaping, electrical stimulation therapy, weight management, intermittent pneumatic compression, low level laser therapy and myofascial release.
Evidence for Management Strategies
Manual Lymphatic Drainage (MLD)
Currently there is very little consensus on the efficacy of MLD due the lack of RCT’s and the variance in techniques performed by practitioners.
A study performed by Tan et al, (2011) recruited 10 patients with Lymphoedema and 12 healthy patients. ICG dye was injected in the arms and legs and participants were observed for 30-60minutes prior to performing MLD, during 20-30 mins of MLD and 30-60minutes after treatment. The findings of this study included:
The number of lymphatic propulsion events as well as the velocity of propulsion was used to measure lymphatic contractile function. According to this study, legs respond better to MLD therapy than arms
There is still considerable individual variation in response to MLD
Lymphoedema patients have significantly poorer lymphatic contractile function in non-affected limbs as well as the affected limb. This supports that secondary Lymphoedema may be the result of predisposition.
Healthy patients response to MLD was greater than Lymphoedema patients, this is thought to possibly be due to increased fibrosis, which impedes lymphatic flow and vessel contractile function
MLD did however demonstrate an improvement in lymphatic contractile function with one treatment in all participants however the degree of response was highly variable (Tan et al, 2011).
Benefits of MLD
Used in areas that can’t be compressed and fluid content will cause problems with function of internal structures including the trunk, male genitals, head and neck
May aid in the prevention of fibrosis and also assist with lymphatic remodelling through influencing direction and flow of fluid within interstitium.
Breast and arm oedema related to cancer treatment is reported to respond well to MLD. Self- massage is also a means of assisting psychologically with the adjustment to their body after cancer
MLD can assist with management of pain, dyspnoea and constipation in palliative cancer patients
Can assist with chronic inflammatory conditions (Williams, 2010).
Has been shown to have benefit post surgically to reduce oedema, improve range of movement and improve surgical outcomes as reported by Ebert et al, (2013) in knee anthroplasty patients
Limitations of MLD
Literature reports that MLD is most effective in mild or early stages of Lymphoedema before there is significant fibrosis. Therefore is most effective used in clients still with pitting oedema, those without pitting would benefit most from compression therapy as a first line treatment (Williams, 2010)
There is still debate as to the safety of MLD with cancer treatment, Williams (2010) states that although for this patient cohort treatment should always be conducted with medical support, there is no evidence clinically or in literature that supports that MLD can promote metastatic cancer.
Cancer patients on cytotoxic chemotherapy may receive delayed treatment with MLD, however palliative management may not benefit from delaying management of oedema which can cause further risk of infection and wounds.
MLD is contraindicated in the presence of acute infection, however if on antibiotic therapy and systemic symptoms have subsided MLD can be resumed (Williams, 2010)
Compression Therapy
Research indicates that compression therapy is superior in the ongoing management of chronic oedema (Elwell, 2015; Gradalski et al, 2015). Compression therapy reduces inflammation resulting from accumulation of protein rich fluid in the limb, reduces oedema through lowered capillary filtration and improved uptake into lymphatics and assist in softening tissue fibrosis (Elwell, 2015). Optimal pressure exerted by compression bandages should be between 30-60mmHg at rest but can having working pressures (during muscle contraction) greater than 60mmHg (Elwell, 2015). There are many types of compression therapy including, elastic and non-elastic bandaging and a plethora of garments from off the shelf to custom designed and adjustable. Compression therapy can aid in reducing limb shape deformity, lymphorrhoea and aid wound repair. It is best to refer clients to a well trained therapist to fit garments or to receive training from the compression garment companies on how to measure for best fit.

Benefits
Improves QoL overall and is effective in managing mild, moderate and severe oedema; early and late onset (Lasinski et al, 2012).
Can be used for patients with active cancer and palliatively
Finding a pressure that is comfortable for the client with compression appears to still be sufficient to have some reduction in volume (ie any compression is better than none)
Limitations
Greatest reductions are seen in the first 5 days, with reductions slowing over the next few weeks and finally reaching a plateau. At this point limb volume can be maintained long term.
Compression must be continued to maintain the reduction in limb volume
Decongestive Therapy
Lasinski et al, (2012) conducted a systematic review of complete decongestive therapy from research conducted between 2004-2011. At that time 99 articles existed however only twenty-six met their inclusion criteria due to small sample sizes, poor study design and insufficient level of evidence, non-english or non peer reviewed. The twenty six studies included were rated as moderately strong in terms of levels of evidence as there were few RCT’s with control groups, well controlled interventions or objective measures of volume, mobility, function and QoL.
Most of the studies also had small sample sizes and limited follow up (12 months or less, some only 24hrs). Most studies looked at the therapies bundled together, i.e. skin care, exercise bandaging and MLD therefore determining which therapy contributed the greatest couldn’t be determined.
The literature that was analysed reported that overall decongestive therapy is effective in reducing oedema, however the efficacy, interrelationship and role of each intervention separately are not well understood (Lasinski et al, 2012). A more recent study by Gradalski et al, (2015) analysed whether the MLD component was essential to decongestive therapy. In this study 26 patients post mastectomy were randomly assigned to either compression bandaging or physical exercise or with the addition of Vodder 30min lymphatic massage to bandaging and exercise. This study found that in the intensive phase there was little difference between the groups, however the compression group did have slightly greater improvement in limb volume reduction.
Skin Changes with Oedema and Skin Care Recommendations
There are very few (to no) studies directly assessing the alterations in epidermal
barrier function with chronic oedema or lymphedema. However it has been observed
that the mechanical stretching associated with oedema, and loss of skin integrity
(micro tearing) impacts greatly on the ability of the skin to resist infection. Poor
vascular and lymphatic flow affects epidermal health and increases hyperkeratosis
which leads to dry and flaky skin. This combined with chronic inflammation in the
tissue can increase pruritus and therefore increase the risk of infection and
mechanical damage due to scratching. Whilst maceration which can occur due to
lymphatic fluid leaking out of the skin is known to increase the risk of skin
breakdown, wounds and ulcerations and increase risk of infection (Minematsu et al,
2011; Flour, 2013).
Chronic inflammation associated with lymphoedema results in a progressive
degeneration of skin tissue the skin becomes hyperkeratotic, the dermis becomes sclerotic, affecting the ECM as well as blood and lymphatic vasculature that becomes increasingly dysfunctional due to tissue changes. Adipose deposition also contributes to increasing size and dysfunction.
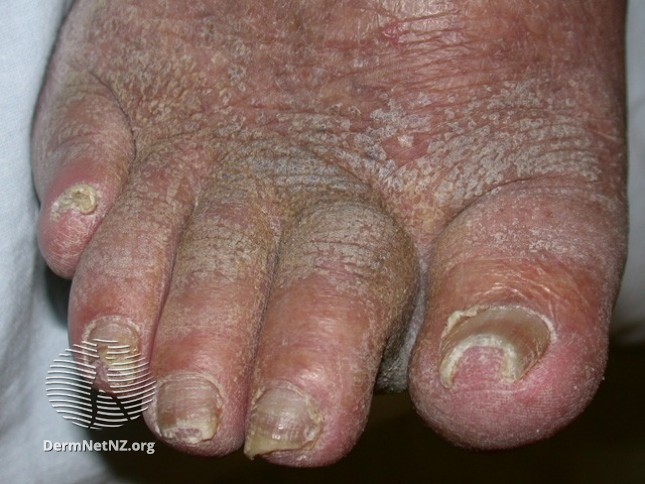
Dermatoses that can present in people with chronic oedema and lymphoedema include
Hyperkeratosis
Fungal Infections
Impetigo
Cellulitis
Intertrigo
Folliculitis
Skin Folds
Viral warts
Ingrown toe nails
Lymphorrhoea
Lymphangiomata
Papillomatosis
Atrophie Blanche
Stasis Dermatitis
Skin cancers (SCC and BCC)
Images obtained from Dermnet NZ https://creativecommons.org/licenses/by-nc-nd/3.0/nz/legalcode
The following should be provided in educating clients that are at risk of developing or have Chronic Oedema and Lymphoedema.
Check the skin regularly for any signs of redness, rashes, bites, bruising, lesions and wounds or generally any changes
Keep the skin clean
Keep the skin dry
Moisturise the skin
Protect against further injury including UV damage, insect bites or other puncture injuries, cuts/abrasions or chemical irritancy.
Maintain a healthy lifestyle and diet
Seek help if something isn't right.
Want to Learn More?
For Dermal Clinicians who wish to further specialise in the area of Chronic Oedema and Lymphoedema management, there are a few programs available for formalised post graduate study or professional ongoing education. Some of these programs require evidence that you currently work in this field.
References
Ebert. J., Joss. B., Jardine. B., & Wood. D. (2013). Randomized trial investigating the efficacy of manual lymphatic drainage to improve early outcome after total knee anthroplasty. Archives of Physical Medicine and Rehabilitation. 94, 2103-2111
Frauziols. F., Molimard. J., Navarro. L., Badel. P., Viallon. M., Testa. R., & Avril. S. (2015). Prediction of the biomechanical effects of compression therapy by finite element modelling and ultrasound elastography. IEE Transactions on Biomedical Engineering. 62(4). 1011-1019
Gradalski. T., Ochalek. K., Kurpiewska. J. (2015). Complex decongestive therapy with or without Vodder II manual lymphatic drainage in more severe chronic postmastectomy upper limb lymphoedema: A randomized noninferiority prospective study. Journal of Pain and Symptom Management. 50(6), 750- 757
Lasinski. B., Mckillip Thrift. K., Squire. D., Austin. M., Smith. K., Wanchai. A., Green. J., Stewart. B., Cormier. J., & Armer. J. (2012). A systematic review of the evidence for complete decongestive therapy in the treatment of lymphoedema from 2004-2011. The American Journal of Physical Medicine and Rehabilitation. 4, 580-601
Tan. I.C., Maus. E., Rasmussen. J., Marshall. M., Adams. K., Fife. C., Smith. L., Chan. W., Sevick-Muraca. E. (2011). Assessment of lymphatic contractile function after manual lymphatic drainage using near-infra red fluorescence imaging. Archives of Physical Medicine and Rehabilitation. 92(5), 756-764
Todd. M. (2013). Chronic Oedema: Impact and management. British Journal of Nursing. 22(11), 623-627
Todd. M. (2013). Self-management in chronic oedema. British Journal of Nursing. 22(12), 701-704
Williams. A. (2010). Manual lymphatic drainage: Exploring the history and evidence base. British Journal of Community Nursing. 15(4), S18-S24








Comentarios